 |
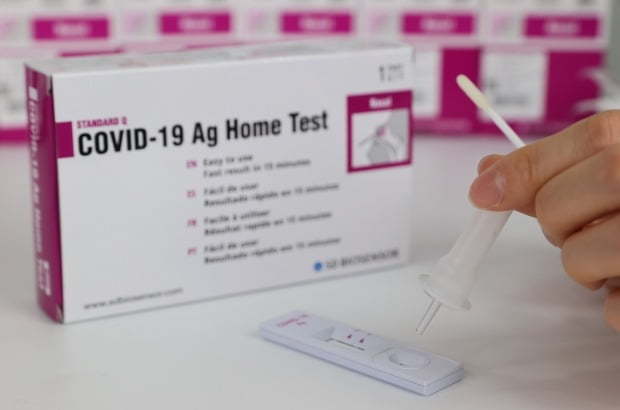
SD Biosensor's COVID-19 self-test kit (SD Biosensor) |
South Koreans may be able to purchase COVID-19 self-test kits as early as next Monday, industry officials said Wednesday.
Coming are two types of home test kits developed by local biotech firms SD Biosensor and Humasis. The test kits will be available at local pharmacies or via online channels, according to the companies.
The rollout comes after the Ministry of Food and Drug Safety last week gave each of them conditional-use approval. The products have been in use in several countries in Europe after they received local approval for emergency use.
Humasis has not yet decided on a retail price, but it would be somewhere between 9,000 and 10,000 won ($8-$9) per unit, the company said. It said the products will hit local shelves on May 3.
SD Biosensor also said the company was in discussions over the price with two local distributors that would supply its test kits in the country.
“(The company) plans to announce the price of its self-test kits on Wednesday or Thursday,” SD Biosensor CEO Heo Tae-young said. “The test kits will be available at local pharmacies, starting early next week,” he added.
According to the Drug Ministry, consumer prices of the self-test kits are expected to reach 10,000 won, as their factory prices are said to be around 7,000 won.
The two home test kits of SD Biosensor and Humasis both use a sterile nasal swab, by which people could detect specific antigens of the COVID-19 present in specimens collected from the nasal cavity. The patients who use the home test kits are able to see their test results in 15 to 20 minutes.
The self-test kits, however, should be used as a supplementary tool, as they are less accurate when compared to the preemptive polymerase chain reaction tests, according to the Korea Disease Control and Prevention Agency. PCR tests are recommended to those who test positive, as well as those who test negative but still show COVID-19 symptoms, the health agency added.
By Shim Woo-hyun (
ws@heraldcorp.com)








