SINGAPORE, April 27, 2022 /PRNewswire/ -- HistoIndex, a global leading artificial intelligence digital pathology (AI-DP) provider, has joined forces with Key Opinion Leaders (KOLs) worldwide in the Nonalcoholic Steatohepatitis (NASH) field to study and validate the reliability of quantifying hepatocyte (liver cell) ballooning injury with its AI-based Second Harmonic Generation/Two-photon Excitation Fluorescence (SHG/TPEF) stain-free imaging and novel qBallooning2 machine learning algorithm. The study highlights the potential of training AI digital pathology with a guided 'Concordance Atlas' to quantify ballooned hepatocytes in a reliable and reproducible manner. The Atlas would then be used to develop and train machine learning AI algorithms in detecting and quantifying the degree of hepatocyte ballooning with higher consistency, sensitivity, and specificity using SHG/TPEF stain-free technology. Findings from this study have recently been published in the Journal of Hepatology.
Teaming up as the qBallooning™ Consensus Group, HistoIndex partnered with 20 renowned hepatologists and hepato-pathologists from the USA, Europe, and the Asia Pacific in addressing the longstanding issue of high inter/intra-observer variation in recognizing the features of ballooned hepatocytes, which affects the diagnosis of NASH as well as the assessment of NASH resolution during clinical trials. Ballooning is one of four key histological features in the diagnosis of NASH, with an appearance of "enlarged" hepatocytes.
The primary goals of the study were:
i) to utilize input from blinded independent assessments by nine internationally recognized expert hepatopathologists in the Consensus Group to generate a dataset of reliably and reproducibly identified ballooned hepatocytes that can be used to support the development of machine learning (artificial intelligence) algorithms for the detection and quantification of hepatocyte ballooning[2] and,
ii) to conduct a focused study that accurately evaluates interobserver variation in hepatocyte ballooning feature recognition. Digitized slides were chosen because they are increasingly used in clinical trials and because only digitisation facilitates the necessary granular annotation of individual cells[2].
One of the study's findings indicated significant varied interpretations amongst expert hepato-pathologists in identifying hepatocyte ballooning in the same digital biopsy images. Therefore, it is highly subjective for pathologists to agree on either the presence or absence of ballooned hepatocytes, particularly in NASH clinical trials. In accordance with FDA clinical trial endpoint requirements, establishing NASH resolution without worsening of fibrosis requires an accurate demonstration of the absence of hepatocyte ballooning[2],[3].
Says Professor Quentin Anstee, Chair of Experimental Hepatology and Consultant Hepatologist, Faculty of Medical Sciences, Newcastle University, UK, joint lead-investigator of the study, "This collaborative deep-dive was an insightful experience for all of us involved in understanding the complexity of how hepatocyte ballooning is perceived. Having an objective and reproducible method to assess hepatocyte ballooning injury is essential in refining and standardizing drug development protocols for NASH, both in terms of clinical trial enrolment and also within the context of a therapeutic efficacy endpoint. Development of assistive AI digital pathology techniques such as those using SHG/TPEF imaging could offer the prospect of a more granular and standardized approach to support drug development."
Development of the Concordance Atlas
Multiple pathologists were able to consistently identify a considerable number of hepatocytes as ballooned or non-ballooned. This paved the construction of an Atlas with consistent annotations of ballooned cells. The annotated image data were used to further refine the qBallooning2 SHG/TPEF-based machine learning AI algorithm for ballooned hepatocyte identification[1].
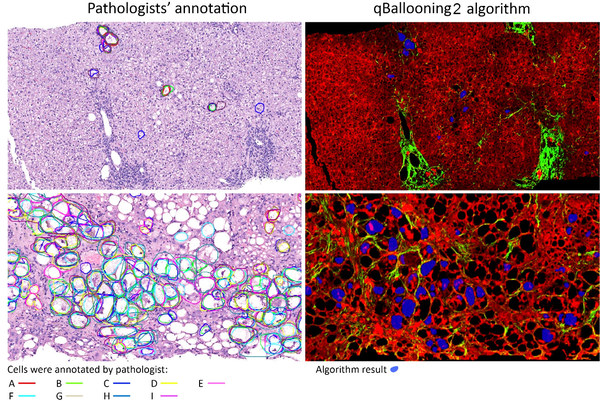
Two examples showing the identification of hepatocyte ballooning by pathologists (left) and qBallooning2 (right). The AI algorithm reading, from a separate drug trial, showing agreement with the study pathologist’s interpretation1. Picture Credits: The Complexity of Ballooned Hepatocyte (BH) Identification: Time to Rethink Trial Endpoints for Nonalcoholic Steatohepatitis? by Elizabeth M Brunt, Quentin Anstee, Dean Tai, et. al. Poster presentation, The Liver Meeting (AASLD) 2021.
Says Professor Elizabeth Brunt, Emeritus Professor, Pathology and Immunology, Washington University School of Medicine in St. Louis, Missouri, USA, joint lead-investigator of the study, "In this study, we trained AI with the most classical ballooned hepatocytes that were agreed on by the majority of the pathologists (5 out of 9), so that AI can identify classical ballooning with a high reproducibility, which is a current limitation for pathologists. Ballooning is a hallmark feature in active NASH. Having a fully quantitative AI approach with SHG/TPEF allows us to consistently identify subsets of hepatocyte ballooning agreed upon by majority of the pathologists, in a standardized manner. Hence, this AI-enabled Concordance Atlas provides the beginnings of a solution to accurately determine intervention efficacy in NASH clinical trials." A second phase for this work is planned to further test and validate the AI accuracy.
Adding to this is Dr. Dean Tai, Co-founder and Chief Scientific Officer, HistoIndex, "I'm delighted to have worked with the leading hepatologists and pathologists from the global NASH community. This study has revealed that AI stain-free digital pathology, specifically SHG/TPEF, is a complementary assistive tool that holds the capability in detecting post-treatment histological changes in hepatocyte ballooning that are clinically relevant and could streamline the way ballooning is interpreted and classified."
The qBallooning Consensus workgroup is an ongoing study, in which the aim is to explore the further development of the Concordance Atlas and AI algorithm, to shed light on additional cellular ballooning characteristics that may be meaningful for efficacy assessment.
Highlights of this study will be presented by Prof. Quentin Anstee during Deciphering NASH: Fibrosis Dynamics in Cirrhotic Patients & Insights into Ballooned Hepatocytes Using AI, a webinar series hosted by HistoIndex and Global Engage on Tuesday, 22 March 2022 at 11.00AM ET/3.00PM GMT. Attendees who wish to register for this webinar may visit the event website in the link above or send an email to info@histoindex.com. Webinar details will also be released on HistoIndex's LinkedIn page.

Seen in this picture are some members of the qBallooning™ Consensus Group, comprising hepatologist and hepato-pathologist KOLs from US, Europe, and Asia-Pacific. The Group was initiated in 2019 to study and validate the reliability of quantifying hepatocyte ballooning injury with HistoIndex’s stain-free AI digital pathology platform with SHG/TPEF and the novel qBallooning2 machine learning algorithm. This photograph was taken in November 2019. Picture Credits: HistoIndex
[Additional Captions for the group picture]
Click on the hyperlinked names of the qBallooning Consensus Group members to view their official affiliations.
Seated (From Left to Right):
Prof. Dina Tiniakos, Prof. Cynthia D Guy, Prof. David Kleiner, Prof. Elizabeth Brunt, Dr. Zachary Goodman, Prof. Aileen Wee, Prof. Caroline Lackner.
Standing (From Left to Right):
Prof. Michael Charlton, Clin Assoc Prof. George Goh Boon Bee, Adj Asst Prof. Leow Wei Qiang, Dr. Stephen Harrison, Dr. Teng Xiao (HistoIndex), Prof. Vlad Ratziu, Prof. Quentin M Anstee, Mr. Anthony Lie (HistoIndex), Dr. Dean Tai (HistoIndex), Prof. Elizabeth Powell, Mr. Ren Yayun (HistoIndex), Dr. Zobair Younossi, and Dr. Brent Tetri.
Consensus Group Members Not in Image:
Prof. Arun Sanyal, Prof. Mary Rinella, Prof. Andrew Clouston, Prof. Matthew Yeh.
About HistoIndex
Founded in Singapore, HistoIndex is a leading MedTech/Healthcare company that specializes in its proprietary integrated stain-free AI digital pathology platform. Enabled by Second Harmonic Generation (SHG) and Two-Photon Excitation (TPE) along with automated imaging analysis algorithms, the integrated platform accurately quantifies histological features and fine measurements that are critical for the evaluation of therapeutic efficacy in clinical trials. The stain-free AI platform is currently involved in multiple FDA clinical trials for Nonalcoholic Steatohepatitis (NASH). In addition, it has benefitted more than 150 research and academic institutes, CROs and biopharma companies around the world in drug discovery and development efforts for fibrotic diseases and cancers.
References
[1] The Complexity of Ballooned Hepatocyte (BH) Identification: Time to Rethink Trial Endpoints for Nonalcoholic Steatohepatitis? |
[2] Complexity of Ballooned Hepatocyte Feature Recognition: Defining a Training Atlas for Artificial Intelligence-based Imaging in NAFLD Elizabeth M. Brunt∗, Andrew D. Clouston, Zachary Goodman, Cynthia Guy, David E. Kleiner, Carolin Lackner, Dina G. Tiniakos, Aileen Wee, Matthew Yeh, Wei Qiang Leow, Elaine Chng, Yayun Ren, George Goh Boon Bee, Elizabeth E. Powell, Mary Rinella, Arun J. Sanyal, Brent Neuschwander-Tetri, Zobair Younossi, Michael Charlton, Vlad Ratziu, Stephen A. Harrison, Dean Tai∗, Quentin M. Anstee∗. J Hepatol. 2022 Jan 25:S0168-8278(22)00024-1. doi: 10.1016/j.jhep.2022.01.011. Epub ahead of print. PMID: 35090960. ∗ Joint senior and corresponding authors. |
[3] Noncirrhotic Nonalcoholic Steatohepatitis with Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry (Draft Guidance) |







![[Today’s K-pop] Blackpink’s Jennie, Lisa invited to Coachella as solo acts](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/21/20241121050099_0.jpg)