Daewoong Pharmaceutical’s liver pill Ursa is under fire for an allegedly misleading TV commercial.
Daewoong on Monday denied accusations brought by a doctors’ group called the Barun Medicine Institute, saying that it was a coincidence that the TV commercial in question had ceased to air.
For future commercials, the company plans to remove a controversial line that purports the efficacy of Ursa for liver patients. A Daewoong representative told The Korea Herald this is being done to avoid unwanted disputes rather than to accommodate claims made by BMI.
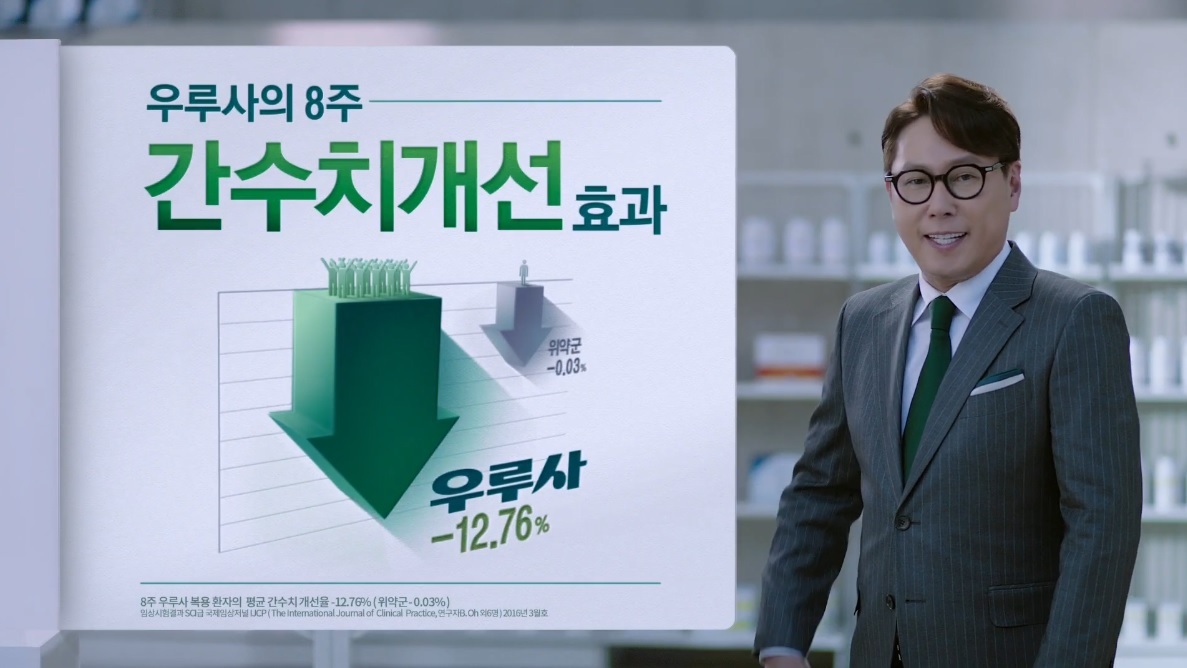 |
The Ursa commercial |
Ursa is one of Daewoong’s iconic drugs that has brought in 33.9 billion won ($29.3 million) in over-the-counter sales for 100-mg pills and 33.4 billion won in prescribed-medicine sales for 300-mg pills, leading to combined sales of 67.3 billion won for the company last year.
Daewoong posted sales of 943.5 billion won in 2018 and net profit of 1.5 billion won. Ursa was recognized by health consultant company Iqvia as the fourth-bestselling OTC drug in Korea.
The Barun Medicine Institute -- started in February 2017 by a group of doctors to research the efficacy of medicinal practices -- says that Ursa lacks clinical research data to prove that it is indeed effective for liver conditions. The institute is located in Jongno-gu, Seoul.
The BMI first brought up the issue with Ursa in late March to the Board of Audit and Inspection. It said that research papers Daewoong filed in 2016 and 2017 lack proof of the drug’s effects related to liver conditions.
The BMI said the TV ad was misleading. “It’s conveniently timed that the Ursa advertisement contract expired (in late May) after we filed a complaint with the authorities,” a BMI representative told The Korea Herald.
Daewoong has rebutted the claims made by BMI, saying that what was lacking in the 2016 paper was substantiated through a 2017 corrigendum. However, the BMI says the corrected version nullifies the original Daewoong paper and invites a bigger issue.
The Daewoong paper, listed in the US National Library of Medicine, has a disclaimer: The safety and scientific validity of this study is the responsibility of the study sponsor (Daewoong Pharmaceutical) and investigators. Listing a study does not mean it has been evaluated by the US Federal Government.
The Ministry of Food and Drug Safety is reviewing BMI’s complaint and could not provide an immediate answer.
Daewoong also argues that the TV advertisement was approved by the Korea Pharmaceutical and Bio-Pharma Manufacturers’ Association as stipulated in clause 80 of article 68-2 in the Pharmaceutical Act.
The KPBMA’s Pharmaceutical Advertising Standards Council comprises university professors and to a certain degree operates independently of the KPBMA.
The council, at the time of Ursa’s TV ad approval, had determined the paper that backs the drug contained adequate information.
By Lim Jeong-yeo (
kaylalim@heraldcorp.com)








