Hypertension drugs gained unwanted attention last year: More than 170 generic drugs sold by 76 local drugmakers were recalled by the Drug Ministry for their inclusion of valsartan, a chemical substance that could raise the risk of cancer.
However, the industry is looking for a rebound this year, with major Korean pharmaceutical firms pushing the envelope in the race for novel hypertension treatments.
The biggest competitor is Boryung Pharmaceutical’s Kanarb, a new hypertension drug developed by a Korean company, which has been sweeping the market.
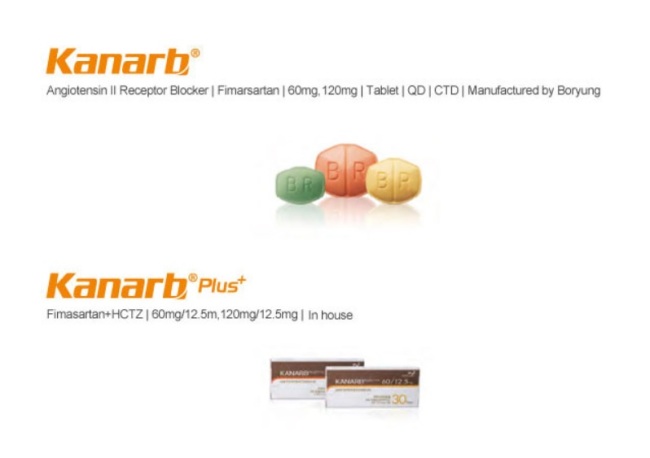 |
Image captured from Boryung Pharmaceutical's website |
Kanarb obtained a domestic marketing license in 2010 as the 15th novel drug from South Korea, and the first hypertension novel drug from the country.
Since then, Kanarb has gone global. It entered the Mexican market in 2011, and has since spread to the rest of Latin America, Russia, Africa and Southeast Asia. Currently, Kanarb has been licensed out to 51 countries and has gained official sales approval in 18 countries.
For Kanarb’s development, Boryung had injected some 50 billion won ($44.3 million) over the course of 12 years. The figure includes 3.5 billion won in government funding. Boryung expects to regain double the investment through Kanarb’s domestic prescription in 2019.
Daewoong Pharmaceutical, on the other hand, announced Thursday that it had completed its lineup of drugs for various hypertension symptoms.
Daewoong currently sells co-promotional drugs Olmetec, Olmetec Plus, Sevikar, Sevikar HC, as well as its self-developed modified drugs Olostar and Olomax.
Olomax is the latest modified hypertension drug from Daewoong. It gained approval from the Korean Ministry of Food and Drug Safety on Thursday.
Olomax is the world’s first novel drug to have amlodipine added to olmesartan and rosuvastatin. The drug can treat both hyperlipidaemia, a high level of cholesterol in blood, and hypertension at the same time.
Each of Daewoong’s drugs tackles different symptoms of hypertension, enabling customized care for patients.
Olomax will launch domestically in May, in two forms according to the density of the rosuvastatin substance, which helps to lower “bad” cholesterol, such as triglycerides and low-density lipoprotein, and raise “good” cholesterol such as high-density lipoprotein in the blood.
Daewoong said it anticipates Olomax to rise in value to become a 20 billion won-worth product in the short term.
By Lim Jeong-yeo (
kaylalim@heraldcorp.com)


![[KH Explains] Can smart chargers ease tensions over EV fires?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/09/22/20240922050108_0.jpg)

![[Online Predators] Online reviews of sex tourism in Southeast Asia proliferate](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/09/23/20240923050595_0.jpg)



