Only a day after Celltrion began distribution of its monoclonal antibody therapy for COVID-19 in Korea, the company announced Thursday that it is setting out to tackle the more contagious South African variant.
“We have a growing library of antibody candidates for COVID-19 variants, of which the ‘32nd antibody’ has shown potency against the South African strain,” said Celltrion’s Honorary Chairperson Seo Jung-jin.
“We will enter animal trial of this 32nd antibody in March, after which we will proceed to take the antibody to South Africa for clinical trials 1 and 2,” said Seo.
The whole process will require six months, according to Seo.
“It was not an easy decision to jump in the fight against COVID-19, because we were amply aware that this will end up a winding fight against variants,” Seo said, in answer to a storm of queries on the efficacy and validity of the currently-approved antibody therapy Regkirona.
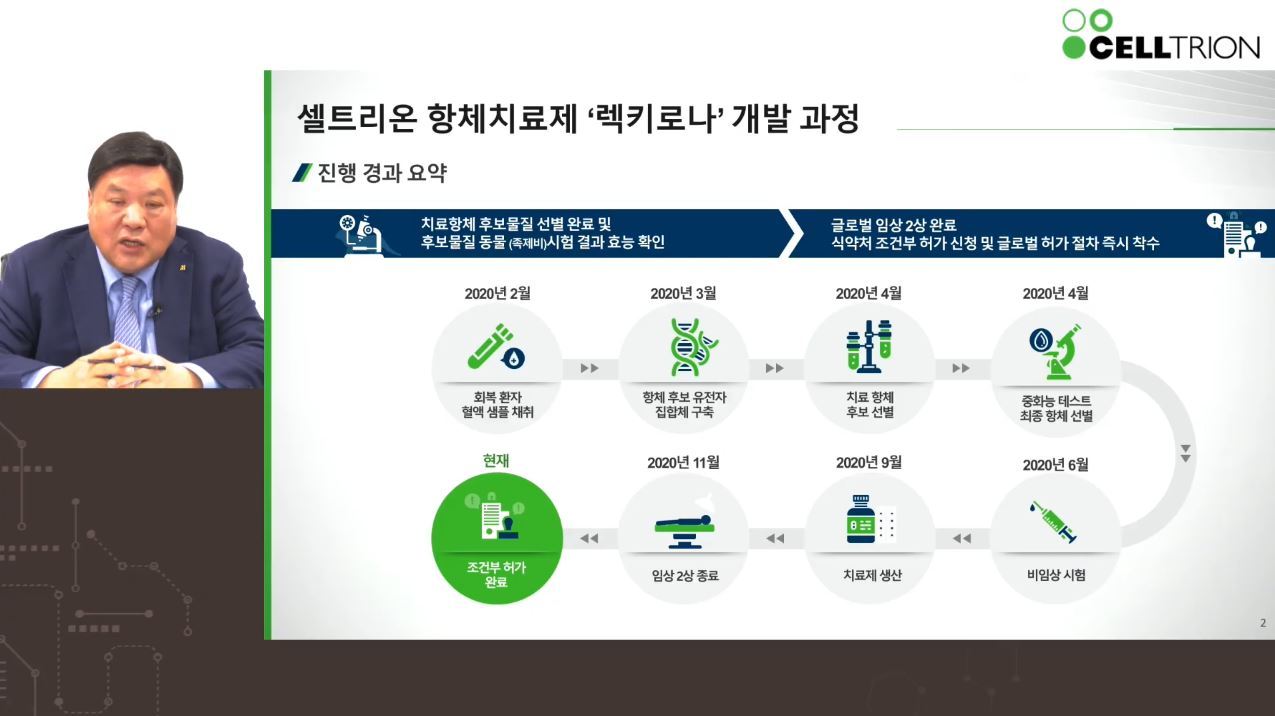
Regkirona (regdanvimab), was given an emergency use approval by Korean drug authorities on condition of submitting their phase 3 clinical trial data within the year.
In its clinical phase 2 trial which targeted 327 Korean and foreign patients in the country and overseas, Regkirona showed a 54 percent clinical improvement rate -- a rate that had disappointed some eager investors.
The therapy, originally developed for COVID-19 patients with mild to moderate symptoms, is now recommended for moderate cases in those 50 and older, due to “lack of economic validation for using expensive antibody therapy for mild cases,” according to Celltrion. For patients with moderate symptoms, Regkirona can deter their progression to more serious cases by shortening the time for recovery and reducing the viral load.
For the 327 patients whose average age was 51, Regkirona could shorten the time to recovery by 3.5 days. Among them, patients with moderate symptoms had taken 5.1 less days to recover compared to the control group and those over the age of 50 experienced 6.4 days curtailed to recovery.
Recovery here meant that the patient no longer posed threat of spreading the virus.
Seo stressed that while ordinary patients’ natural immune system helps them recover within three to four weeks’ time, during that process their internal organs could be impaired. Regkirona could prevent such stress on the body, Seo said.
Antibody therapies, because of their costly raw resources, are naturally on the more expensive side among various types of medical remedies.
In Korea, the government has paid for Regkirona for state-designated COVID-19 hospitals to receive necessary supplies for free.
Celltrion has enough Regkirona to go around to 100,000 patients in the nation, and expects to exhaust the lot here.
“We have supplied Regkirona at base rate without a margin to the government, under the belief that COVID-19 treatments are public goods for the people of this nation,” Seo said.
Overseas, Celltrion strategizes to supply Regkirona at one-fifth of the cost of its US competitors, Seo said. The volume of drug production will depend on the demand.
US’ Eli Lilly and Regeneron have developed monoclonal antibody cocktails for COVID-19 treatment in patients with mild to moderate symptoms. Theirs have already gained the US Food and Drug Administration’s emergency use approval in November 2020 and are under review with the European Medicines Agency since February.
Although Celltrion will be a latecomer in those markets, its competitive price could cause a ripple in the market.
Celltrion is undergoing pre-application discussions with the FDA and EMA.
The company is also researching on different types of drug delivery that could help reduce production costs. This could mean that the company is looking at delivering the drug through subcutaneous injections.
“COVID-19 will not end this year,” Seo said, “We are making efforts to reduce cost in production of COVID-19 therapy, as we believe it will become an infectious disease that returns every year like seasonal flu.”
It has taken Celltrion a year to provide Regkirona to the public; 10 months to develop the therapy and an additional two months to gain emergency use approval, under Seo’s leadership and the efforts from Celltrion employees working around the clock in dangerous regions with high SARS CoV-2 infection rate.
Seo urged investors to think of its COVID-19 therapy as public goods and not a point of interest for investment purpose.
“Please know that our efforts are directed to secure South Korea’s pharmaceutical sovereignty,” Seo said.
“We wish for investors to refrain from making hasty investment decisions based on the sensational effects of our COVID-19 therapy development but instead focus on the promises of our main biosimilar products,” Seo said.
Seo, who founded and led Celltrion through its two-decade history, will retire in March.
“As Honorary Chairperson, my involvement in the company will be minimal. I will not ask for a salary or an office -- but I may offer my service as a fire truck when the company needs it,” Seo said.
By Lim Jeong-yeo (
kaylalim@heraldcorp.com)