Celltrion, a South Korean biopharmaceutical company, is making headway in a newly emerging category of medicine known as biosimilars -- lower-cost copies of brand-name biologic drugs that have lost patent protection, around the world.
The Korean drugmaker on Monday said it has gained approval from Australia to begin selling Remsima -- its biosimilar replication of the world’s third best-selling rheumatoid arthritis treatment drug Remicade.
U.S.-based global pharmaceutical firm Hospira, Celltrion’s marketing partner, will take charge of the drug’s marketing, distribution and sales operations.
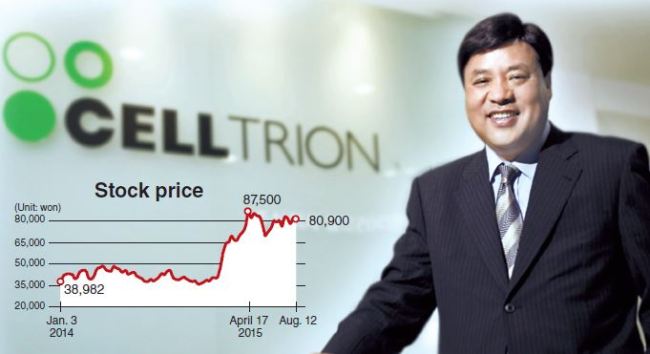 |
Celltrion chairman and CEO Seo Jung-jin (Celltrion) |
“Pricing greatly influences sales in Australia,” said a Celltrion official. “Given this, cheaper biosimilars (like Remsima) are set to hold a competitive edge in the price-sensitive Australian pharmaceuticals market.”
Industry watchers expect Celltrion to quickly gain a strong market share in the country, which “is favorable to low-cost biosimilars given that the government mandates firms to publicly share their drug pricing to promote price competition,” according to Eugene Investment & Securities analyst Shin Jae-hoon.
Celltrion is the top-listed company on the Korean tech-savvy KOSDAQ market. Boasting a market value of around 9.94 trillion won ($8.4 billion), the firm regularly records high trading volumes.
The Korean firm is also considered an early pioneer in increasing competition in the global biopharmaceutical market long dominated by big-name drugmakers such as Roche, AbbVie, Novartis, Pfizer and Johnson & Johnson.
Whereas traditional chemical-based drugs can easily be copied by generic manufacturers when their patent expires, biologic treatments are much harder to replicate and involve intensive R&D, which has allowed big firms to maintain their monopoly till recently.
Today, a number of biosimilar developers are shaking up the global pharmaceutical industry by introducing effective yet low-cost alternatives to expensive biotech drugs, many of which have reached or are nearing their patent expiration dates.
Celltrion, joining hands with Hospira, set a milestone in the pharma industry in February by kicking off sales of its biosimilar drug Remsima -- also known as Inflectra -- across Europe, including in Britain, France and Germany.
 |
Celltrion’s biosimilar drug Remsima, a copy of Johnson & Johnson and Merck & Co.’s anti-inflammatory biologic drug Remicade. (Celltrion) |
The introduction of the biosimilar, which is up to 45 percent cheaper than the original, has already led to a decline in regional sales of the original drug sold by the U.S.-based Johnson & Johnson and Merck & Co.
Faced with new competition in Europe, Johnson & Johnson saw its second-quarter sales of Remicade outside the U.S. fall to $580 million, compared to $783 million a year earlier.
Merck, which handles the marketing of Remicade in Europe, saw sales of the drug decline by 25 percent to $455 million in the second quarter, as more European doctors reportedly chose to prescribe the cheaper version of the drug.
Driven toward expansion, the Korea-based biosimilar producer has also set its sights on entering the United States -- the world’s largest pharmaceuticals market.
The company is currently awaiting official approval from the U.S. Food and Drug Administration, which, in a landmark decision in March, gave its first-ever sales approval for a biosimilar copy of Amgen’s well-known cancer treatment Zarxio, developed by Novartis AG.
Meanwhile, biosimilars are forecast to become a multibillion-dollar industry in the years ahead, bringing sweeping changes to the global pharmaceuticals sector.
Citigroup analyst Andrew Baum in February predicted that, “(Original drug developers) will likely lose an aggregate $360 billion in revenues over the next 10 years, with roughly $110 billion captured by biosimilar sponsors,” generating about $50 billion in savings for health care systems.
Eyeing such growth potential, the Korean government has been stepping up its efforts to support the nation’s biosimilar industry as a future growth engine. Earlier this month, the ICT and health ministries announced plans to inject 40 billion won into nurturing high-tech biopharma firms over the next three years.
Alongside Celltrion, Samsung Bioepis and Hanwha Chemical are among the major Korean players looking to take up a significant share of the burgeoning field of biotech medicine.
By Sohn Ji-young (
jys@heraldcorp.com)







![[Exclusive] Hyundai Mobis eyes closer ties with BYD](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/25/20241125050044_0.jpg)
![[Herald Interview] 'Trump will use tariffs as first line of defense for American manufacturing'](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/26/20241126050017_0.jpg)
![[Herald Review] 'Gangnam B-Side' combines social realism with masterful suspense, performance](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/25/20241125050072_0.jpg)