South Koreans with Allergan’s textured breast implants are expressing fear and complaining about a lack of direction from authorities, a week into the latest developments surrounding the first Korean patient being diagnosed with breast implant-associated anaplastic large cell lymphoma, or BIA-ALCL, a rare cancer of the immune system.
The first BIA-ALCL diagnosis in Korea was publicly announced Wednesday last week by the Korean Ministry of Food and Drug Safety. It was the fourth such case in Asia, following previously reported diagnoses in Thailand and Japan.
The US Food and Drug Administration reported Allergan products had six times greater chances of leading to BIA-ALCL than other rough-surfaced breast implants from different manufacturers.
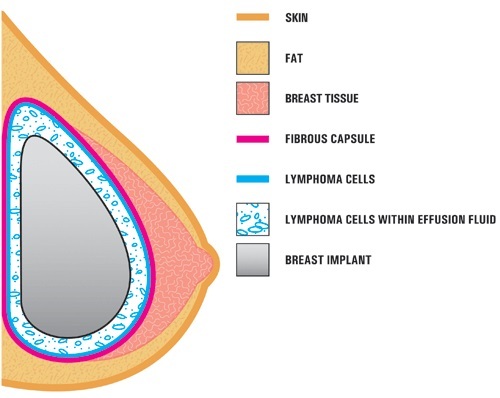
Researchers suspect continuous inflammation from rough-surfaced implants may lead to transformations in tissue cells that become immune system cancer. Allergan’s biocell textured implants have among the coarsest surfaces, which local doctors now suspect to be the cause of BIA-ALCL.
 |
(US' Food and Drug Administration) |
The US FDA said of 573 unique global cases of BIA-ALCL, 481 were attributed to Allergan implants. Of 33 recorded patient deaths, 12 of the 13 patients for whom the manufacturer of the implants was known were confirmed to have Allergan breast implants.
Allergan issued a voluntary global recall of unused Natrelle Biocell Textured Breast Implants -- both saline and silicone types -- from clinics on July 24.
With a confirmed diagnosis of BIA-ALCL in Korea, the issue affects this country too.
According to a report from Rep. Choi Do-ja of the National Assembly’s Health and Welfare Committee, Korea distributed around 222,500 textured breast implants in the 12 years between 2007 and 2018. Allergan’s textured product accounted for 50 percent of the total, more than 114,000.
With a lack of direction from local authorities on the matter, and limited information available on the internet, Korean patients have started storming local plastic surgery clinics.
“I had been assured Allergan’s textured implants were the best that were available. The doctor who treated me told me he would recommend them to his family,” said a 24-year-old patient surnamed Lim, who was visiting a plastic surgery clinic in Gangnam to check on the implants she got three years ago.
 |
A patient with Allergan Biocell textured breast implant speaks to The Korea Herald. |
“Even if I were to undergo another surgery to replace these implants I wouldn’t know how to trust alternative products, because these were what I was told were the best then, and see what happened.”
Lim said she had received no direct guidance from authorities.
Another patient, a 27-year-old surnamed Choi, echoed the sentiment. Choi had her surgery in 2015.
“I didn’t know what to do after the news broke, or which ‘big’ hospital I should go to,” Choi said, adding that she relied on word of mouth to come to a clinic in Gangnam for a checkup, where she was told nothing was wrong. Choi said she will wait until further notice from authorities but said if she could, she would like to change her implants for a safer option.
The Drug Ministry, which oversees approvals for medical devices and pharmaceuticals in Korea, said it is in talks with Allergan to determine the next course of action, including compensation for patients. The recalled implants, better known in Korea as “water drop” breast implants, were the most popular kind for the past 10 years.
 |
Barn Jae-sang, chief surgeon at Banobagi Plastic Surgery Clinic in Gangnam, Seoul (The Korea Herald) |
Barn Jae-sang, a surgeon at Banobagi Plastic Surgery Clinic in Gangnam, Seoul, who has operated on breasts for 19 years, said about 500 to 600 breast implants are used at his hospital in a year for Korean and foreign patients alike. From the hospital database, he learned that about 4,200 Korean patients had textured breast implants over the last decade, not limited to Allergan products. Banobagi alerted these patients to potential risks and advised them to visit the hospital for a follow-up, he said.
Macro-textured implants, the type that Allergan manufactures, pose a bigger concern outside of the US, as they only represent about one-tenth of the overall breast implant market in the country but as much as 80 percent elsewhere, according to the US FDA.
Prior to the FDA announcement, France, Canada and Australia had already initiated recalls of certain textured breast implants, including Allergan’s.
The Drug Ministry here told The Korea Herald that it had also been aware of the BIA-ALCL cases since the US FDA first uncovered the problem in 2011, but that the risk of disease was too low, especially for Asians, for authorities to investigate the matter or act upon it.
For those without obvious symptoms, the FDA does not recommend removal of implants, although the agency advises that all breast implant patients, regardless of what product they use, should be aware of the risks of BIA-ALCL.
 |
Minn Kyung-won, professor emeritus at Seoul National University Hospital, speaks to The Korea Herald. Types of breast implants are displayed on the table. (The Korea Herald) |
Minn Kyung-won, professor emeritus of Seoul National University Hospital and a fellow of the American College of Surgeons, said the Drug Ministry and Allergan must include the voices of private practice physicians who hold the most patient data when deciding on compensation methods.
The reason the textured implants are popular outside the US, professor Minn says, is that the surgery is delicate and renders more natural-looking results. The implants, although they’re called rough-surfaced, are still soft to the touch. Yet the uneven surface is more cohesive against the inner breast tissue, assuring it doesn’t spin inside the body and preserves its hanging, water drop-like shape. The preceding generation of smooth implants moved more easily inside the body and had to be regularly massaged.
“It’s difficult to put a finger on one party to blame for this aesthetic-centered surgery,” Minn said, “But the patients who decide to have breast augmentation usually monitor their body closely on a daily basis, which allows them to quickly detect and report anomalies, bringing prompt attention to any issue.”
An individual’s risk of developing BIA-ALCL is considered to be low, according to the FDA, but as the cancer can lead to death, authorities advise patients to monitor their breasts and visit the hospital once every six months even without symptoms. In most patients, BIA-ALCL can be successfully treated, the US FDA said.
By Lim Jeong-yeo (
kaylalim@heraldcorp.com)











