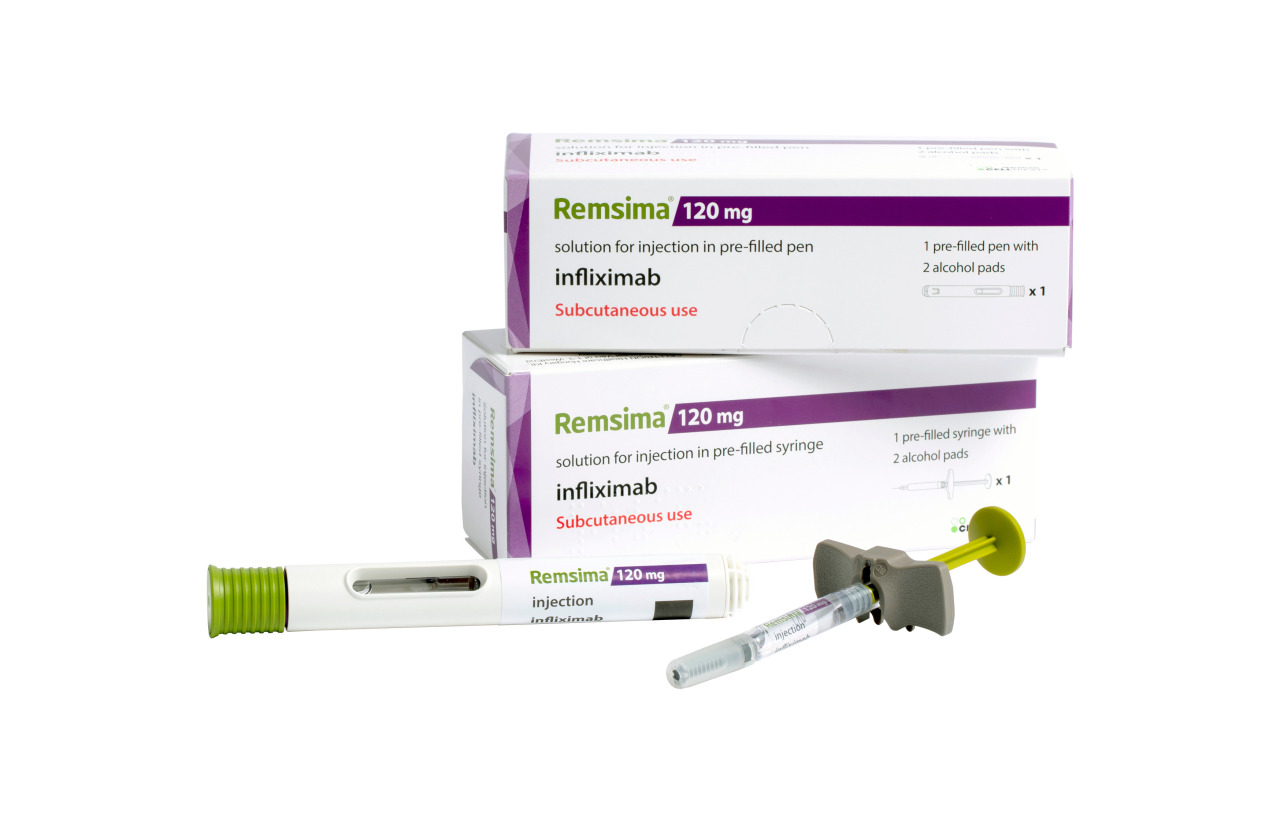
Subcutaneous rheumatoid arthritis treatment Remsima SC launched in the Netherlands, its third European market, Celltrion Healthcare said Thursday.
Remsima SC is an under-the-skin injection of infliximab biosimilar that treats autoimmune diseases. So far, it is approved to treat RA indication, and is still in the process of adding inflammatory bowel disease indication.
Celltrion Healthcare said it inked a partnership with the biggest Dutch private health insurance company CZ, who will return all Remsima SC costs for patients.
Tom Huizinga, the head of Rheumatology Department at Leiden University in Netherlands, said that Remsima SC provides a dual formulation option for doctors who may want to switch back and forth accordingly between intravenous infliximab injection, which must be administered by a medical professional, and subcutaneous injection, which the patients can self-administer at home.
In Europe, Remsima SC is distributed in Germany, the UK and the Netherlands.
By Lim Jeong-yeo (
kaylalim@heraldcorp.com)








