 |
Yang Eun-young, vice president of Samsung Biologics' CDO business (left) and John Gill, director of cell line development (Image screen-captured from YouTube live presentation) |
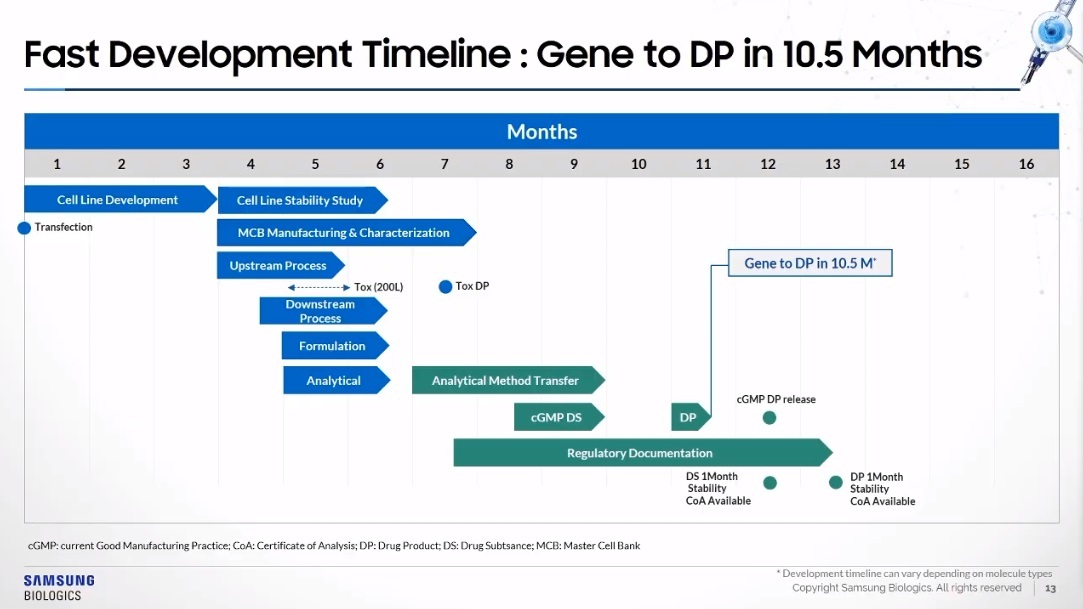
The cell line development process is a significant phase within the drug life cycle, as these cells act as the living “factories” for biopharmaceutical drugs.
The S-CHOice cell line stands out for having a high productivity of over 7-gram titration per liter and maintaining cell viability higher than 90 percent through day 21 in the manufacturing process, said Gill, the director of cell line development.
The CHO in “S-CHOice” stands for “Chinese hamster ovary.” Samsung Biologics scientists screened thousands of clones looking for the one that was friendliest to being manufactured, and isolated those that had doubling times of between 18 and 20 hours, leading to a faster timeline from transfection to research cell bank to guarantee high productivity and viability, Gill said.
“The quality and safety of the therapeutics start from cell line. The main focus of the cell line development aspects here, are looking at product quality through the entire life cycle, assuring monoclonality as regulatory guidance and additionally looking at high and consistent process yield,” Gill said.
Samsung Biologics, founded in 2011 as a contract manufacturing organization for finished biologics drug products, expanded to contract development in 2018. Since then, it has assisted global clients in reaching investigational new drug and biologics license application filings in 55 cases.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)







