 |
(Yonhap) |
Three South Korean COVID-19 test-kit companies have obtained preliminary approval from the US Food and Drug Administration (FDA), the foreign ministry said Saturday.
The initial FDA approval will allow the Korean test-kit makers to sell the products in the United States, where the number of confirmed coronavirus cases has surpassed 100,000, an official at the Ministry of Foreign Affairs said over the phone.
The official did not provide the names of the three approved test kits or a time frame for their sales in the US.
The ministry expected the US emergency-use authorization for the South Korean test devices to pave the way for the kits to be exported to the US market.
In a phone conversation with President Moon Jae-in on Tuesday, US President Donald Trump expressed hope that South Korea could provide the medical devices to help contain the spread of the respiratory illness in the US.
Moon replied that his government would provide "maximum support" if available as the two leaders discussed bilateral cooperation in the fight against the novel coronavirus pandemic.
He then pointed out that approval by the FDA might be required.
In response, Trump said he would make immediate action for that "within today."
South Korea has been widely lauded for its effective and swift response to the spread of the virus, taking advantage of its advanced testing capabilities. It has been exporting test kits to a number of countries.
According to the ministry, 47 countries have so far asked about importing South Korean-made coronavirus test kits, while an additional 39 countries have requested them as humanitarian aid.
The number of such requests from foreign countries is likely to increase down the road, as South Korea has been recognized globally for its diagnostic capabilities that led to a slowdown in new COVID-19 cases, industry insiders said.
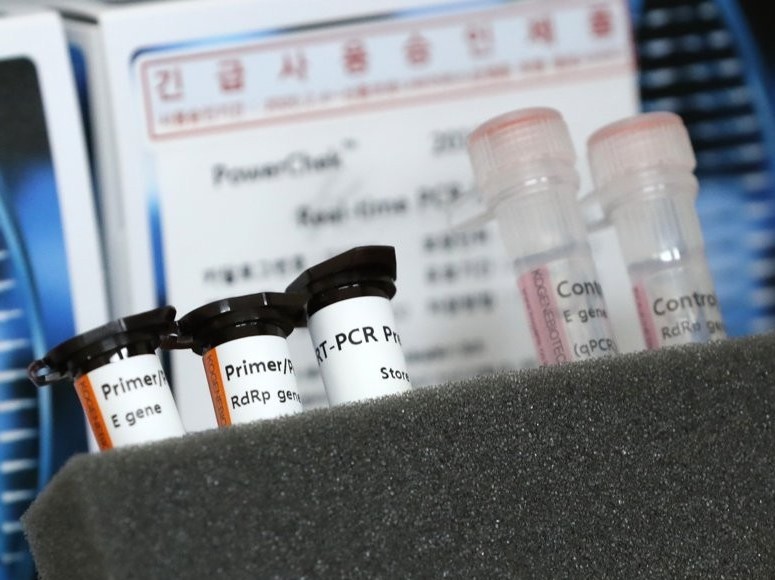
So far, five local biotech firms' kits have earned emergency-use approval from Seoul's health authorities. They are Seegene's Allplex, Kogene Biotech's Power Check, Solgent's DiaPlex Q, SD Biosensor's Standard M and BioSewoom's Real-Q.
All are based on the real-time polymerase chain reaction (RT-PCR) testing method, which produces a result in as little as six hours.
The companies now make enough diagnostic reagents to test 135,000 people a day.
Seegene said 95 percent of its COVID-19 test reagents are already being exported to foreign countries.
Meanwhile, the FDA on March 28 (local time) gave emergency authorization to a portable test kit from US health care company Abbott Laboratories that can determine within minutes whether a person is infected with COVID-19, according to foreign newswires. (Yonhap)







![[Weekender] Korea's traditional sauce culture gains global recognition](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/11/21/20241121050153_0.jpg)
